Abstract
Introduction: There is growing interest in the role of Epithelial to Mesenchymal Transition (EMT) regulators in immune cell development, but the functional role of EMT in B-cell biology and transformation remains obscured. The outcomes of the "classical" EMT activation of epithelial cells are known; committed migratory cells do not divide and become resistant to apoptotic stimuli. Importantly, EMT supports migratory cells' "stem cell-like" properties, allowing differentiation into more than one cell type and/or terminal differentiation and senescence. Chronic lymphocytic leukaemia (CLL) cells engage with antigen and microenvironment stimuli in tissue sites that lead to proliferation, followed by their egress from the tissue sites into the peripheral blood. This lifecycle implies functional changes altering CLL cell movement. Here we show EMT-like activation in CLL that correlates with disease progression and transformation. We identified a targetable candidate and show that it can be inhibited by small molecule ligands.
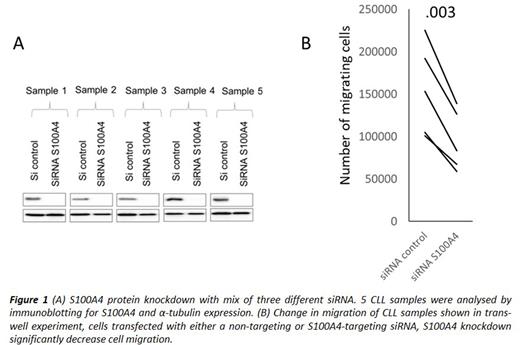
Results: Our analysis of available expression datasets showed the expression of various EMT factors in healthy B cells. The single-cell analysis of lymph nodes confirms strong EMT-like activation in healthy B-cells (GSE159929), confirming the idea that genes involved in classical EMT would be expressed differently depending on the stage of B-cell differentiation. The following GENEVESTIGATOR analysis of B cell malignancies highlighted high EMT-like activation in CLL cases. However, differential expression (DE) analysis of peripheral blood (PB) samples from 44 CLL patients and 24 healthy B-cell donors identified six EMT factors aberrantly expressed in CLL cells compared to healthy B cells indicating significant changes in CLL EMT-like activation. At the transcription level, ZEB1 expression was higher and ZEB2 expression lower in PB CLL cells. The main stimulatory events in CLL cells appear restricted to affected tissue sites, primarily in lymph nodes (LN) where CLL cells migrate to reactivate and proliferate. Further, EMT factor protein expression was confirmed in CLL LN tissue microarrays. Strikingly, transformation into a more aggressive form (Richter syndrome) strongly correlates with an increase in ZEB2 but not ZEB1 protein expression. Interestingly, analysis of the published transcriptome data set of CLL cells co-cultured with nurse-like cells shows an increase in ZEB2 and a decrease in ZEB1 expression after 14 days (GSE13811). An apparent reliance of the EMT-like activation in CLL on the environmental stimuli has been demonstrated in CLL cells stimulated with BCR activation (anti-IgM) or mitogen (PMA), implying protection from cell death and activation of migration. Additionally, we observed higher S100A4 expression in CLL cells compared to mature B-cells from healthy individuals. Previously it has been shown that this calcium-dependent protein can control cell movement by direct interaction with non-muscle myosin and via regulation of stress fibre formation, thus facilitating cellular protrusions and increasing cell migration and invasion. Our analysis has shown the S100A4 positive correlation with CLL markers of poor prognosis, Zap70 and CD38. The S100A4 knockdown with siRNA dramatically impeded CLL migration in trans-well assay (Figure 1). Biochemical inhibitor screening for disruption of S100A4 interaction with myosin II identified several promising leads active in the low micromolar range in both biochemical and cell assays.
Conclusions: Our findings improve the understanding of CLL pathobiology. Normally, healthy naïve B cells give rise to multiple B-cell subtypes with specific functions and locations. EMT-like activation likely contributes to B-cell development at different stages of differentiation and B-cell malignant transformation. Our investigation shows that the EMT-like activation is dysregulated in CLL cells compared to healthy B cells. The EMT expression signature changes upon B-cell receptor stimulation. These results suggest that targeting some of the aberrantly expressed EMT components such as S100A4 in CLL can be exploited, prolonging remission by, for example, impeding CLL returning to the tissue sites after an egress due to the treatment with Ibrutinib depriving these cells of vital microenvironmental support.
Disclosures
Gribben:Amgen: Honoraria; AstraZeneca: Honoraria, Research Funding; Celegene: Research Funding; Roche/Genentecg: Other: Personal fees; AbbVie: Honoraria, Other: Personal fees; Janssen: Honoraria, Other: Grant and personal fees ; BMS: Honoraria, Research Funding; Gilead/Kite: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal